Revista Industrial Data 27(1): 7-23 (2024)
DOI: https://doi.org/10.15381/idata.v27i1.25643
ISSN: 1560-9146 (Impreso) / ISSN: 1810-9993 (Electrónico)
Analysis to Increase the Recovery and Quality of Zinc Concentrate in Polymetallic Ore Flotation in the Yarusyacán District, Cerro de Pasco
José Barrientos Ríos[1]
Luis Quispe Gallegos[2]
Vidal Aramburú Rojas[3]
Jorge Ortiz Barreto[4]
Ronald Quispe Castro[5]
Rosa María Tiburcio Alva[6]
Wendy Choque Perez[7]
Design and Technology
Submitted: 29/09/2022 Accepted: 30/08/2023 Published: 21/06/2024
DOI: https://doi.org/10.15381/idata.v27i1.23709.g20437
ABSTRACT
Ore from the Yarusyacán District was analyzed in this research study, revealing the presence of sphalerite type 2, with a particle size ranging from 35 to 150 microns. Other species found included quartz, pyrite, pyrrhotite, calcium and potassium silicates, calcium carbonates, galena, chalcopyrite, and iron hydroxides. Based on the mineralogical characterization, laboratory tests were conducted that showed that dosing a secondary collector known as cuprocyanide can control the recovery and quality of the zinc concentrates in the third cleaning feed. Results showed that a 35 g/t dosage of cuprocyanide recovered 73.80% of zinc, whereas a 50 g/t dosage recovered only 52.94%. This demonstrated that both zinc grade and recovery increase when cuprocyanide is dosed in greater quantities; however, the excess of this reagent tends to decrease said values.
Keywords: cuprocyanide, metallurgical factor, zinc recovery.
INTRODUCTION
Flotation is a metallurgical process for mineral concentration based on the principle of hydrophobicity, which is one of the main characteristics of sulfide species. When these species come into contact with water and air, they move toward the surface as froth (Azañero et al., 2010; Utepbaeva et al., 2023). Zinc is one of the metals that benefits the most from this process. In 2021, Peru produced 1,533,135 fine metric tons (FMT) of zinc, representing a 14.8% increase compared to the previous year. The majority of the production value was attributed to mining companies such as Antamina, Volcan, and Nexa Resources, which were responsible for 34.8%, 9.3%, and 7% of the total zinc production, respectively (Ministerio de Energía y Minas, 2021). These figures position Peru as the second-largest zinc producer in the world, surpassing Australia (U.S. Geological Survey, 2022). However, current reports suggest a year-on-year decrease of 13.2% in zinc production in January 2022 due to the geological complexity of processing these types of ores (Inga et al., 2020). The global health crisis significantly impacted zinc production in Cerro de Pasco, leading to a 32.5% decrease in mining production at the departmental level (Instituto Peruano de Economía, 2022). Despite this, the city of Pasco remains one of the main producers of zinc in Peru.
The complexity of the sphalerite flotation process is influenced by various factors. These factors include the type and degree of liberation of mineral species present in the ore, as well as associated minerals, lattice impurities, particle size and shape, mineral dissolution, and liberation of inclusions. As for the beneficiation process, efficiency is determined by various factors including values assumed for the pulp dilution variables, types of reagents used, quality of water, pH, and ore particle size, among others (Tang et al., 2023).
By adding copper sulfate, sphalerite is activated and floats effortlessly with the addition of a xanthate-type collector and a frother. This process works best for pH values ranging from 6 to 12 (Mateos, 2020; Yu et al., 2019). However, mineral species can also float with most sulfhydryl-type collectors at acid pH values. Collectors have also been developed for the flotation of sphalerite without activation, such as synthetic reagents (Natarajan & Nirdosh, 2006).
This research study focuses on evaluating new collectors for sphalerite flotation to improve the recovery and quality of zinc concentrate, taking into account the complex and ever-changing characteristics of the ores found in the Yarusyacán District, Cerro de Pasco (B. Li et al., 2017). The study evaluates the dosage of cuprocyanide collector for obtaining favorable results, measured in grams per ton (g/t). The findings of this research will be beneficial for companies working with similar mineralogical species or looking to optimize their flotation process based on the parameters established in this paper.
Objective
The main objective of this research study is to identify a suitable reagent for the flotation process currently being carried out in the concentrator plant located in Yarusyacán District, Cerro de Pasco, to make the exploitation of these deposits feasible.
Hypothesis
The use of reagents during the cleaning stage in zinc flotation increases the grade and recovery of zinc concentrate obtained in the metallurgical plant in Cerro de Pasco.
Justification
This research was conducted to address the processing issues related to polymetallic sulfide ores, which are commonly encountered in concentrator plants. The displacement of copper minerals in the zinc flotation circuit is a major issue that leads to low recovery and poor concentrate quality, which does not meet commercial standards. A sequential analysis was carried out to observe the response of different metals to the flotation process. By applying an established objective, the comparison provides more significant results in percentage terms, particularly in the case of zinc standard concentrate, where higher percentages are expected. The findings of this research suggest that the cuprocyanide collector can be applied to solve this problem in plants that process ores with similar mineralogy.
Background
· Palafox et al. (2010), in their research paper Rediseño del circuito de flotación de zinc usando modelación matemática, found that modifying the circulating loads in the flotation process increased zinc recovery from 93% to 96.7%, while maintaining the concentrate grade at a constant 57%.
· J. Li et al. (2017), in their research Effect of Combined Reagents of Sodium Citrate and Sodium Pyrophosphate on Flotation Separation of a Polymetallic Lead-Zinc Ore, demonstrated the effectiveness of the combination of pyrophosphate and sodium citrate in lead and zinc recovery. The authors reported recovery rates of 81.31% and 93.11%, respectively.
· Xu et al. (2022), in their paper A Comprehensive Recovery Process for Selective Separation and Enrichment of Copper, Zinc and Iron Minerals from a Polymetallic Ore and the Adsorption Mechanism of Collector Z-200, analyzed a polymetallic ore with a grade of 0.61%, 1.68%, and 14.17% Cu, Zn, and Fe, respectively. By using collector Z-200 in Cu flotation and BX reagent in the Zn stage, they obtained recoveries of 86.1%, 87.6%, and 77,8%, respectively.
· Kyaw et al. (2021), in their research paper Improvement of reagent flotation modes of sphalerite and pyrite from deposits of copper-zinc pyrite, polymetallic copper-zinc pyrite and polymetallic ores, evaluated the flotation of sphalerite and pyrite in an alkaline medium. They used copper sulfates as an activator and potassium butyl xanthate in pH ranges from 8 to 12. They were able to recover just under 80% of zinc in the most representative tests.
METHODOLOGY
The research conducted is experimental, qualitative, quantitative, and deductive. A metallurgical test design was developed based on the identification of mineralogical species, their distribution in the sample, and liberated particles. Numerical data was collected and interpreted to understand the relationship between cuprocyanide dosage and the recovery and quality of zinc concentrate (Arias & Covinos, 2021).
RESULTS
The tests conducted identified the presence of several mineral species, including pyrite, pyrrhotite, sphalerite (blende-marmatite), chalcopyrite, intermediates ores, galena, magnetite, and marcasite. The bonds present between the species, the volumetric distribution, and the degrees of liberation were analyzed through polarized optical microscopy and electron microscopy (see Table 1).
Figures 1, 2, 3, and 4 show the main microphotographs of the representative zinc concentrate.
Table 1. Minerals and Associated Minerals Found in the Sample.
|
Mineral |
Associated Minerals |
Volumetric Distribution % |
Degree of Liberation % |
|
Gangues (GGs) |
cp, py, mt, sph2, po, gn, sp1, hm, mc |
63.00 |
98.29 |
|
Sphalerite1 (sph1) |
cp, gn, po, interm. ores, GGs, py |
6.39 |
87.41 |
|
Galena (gn) |
sph1, GGs |
0.75 |
55.56 |
|
Pyrite (py) |
GGs, mt, cp, po, LIMs, sph1, mc |
13.12 |
91.96 |
|
Pyrrhotite (po) |
sph1, py, GGs, cp, interm. ores |
3.22 |
79.73 |
|
Sphalerite 2 (sph2) |
GGs |
0.40 |
71.43 |
|
Chalcopyrite (cp) |
GGs, sph1, py, po, interm. ores |
1.26 |
88.37 |
|
Marcasite (mc) |
GGs, py |
0.24 |
100.00 |
|
Intermediate ores (Interm. Ores) |
sph1, po, mt, py |
0.90 |
76.97 |
|
Magnetite (mt) |
GGs, hm |
4.63 |
85.59 |
Source: Buenaventura Ingenieros S.A. (2014).
|
|
|
|
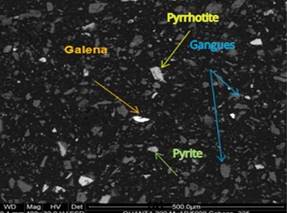
Figure 1. Liberated particles of po, py, gn, and GGs (magnified at 400x). |
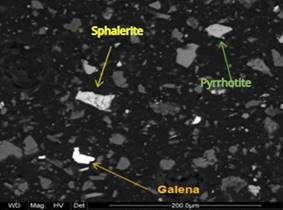
Figure 2. Liberated particles of sph, py, and GGs (magnified at 800x). |
|
|
|
|
Figure 3. Liberated particles of sph, gn, and GGs (magnified at 800x). |
Figure 4. Liberated particles of gn, sph, and po (magnified at 800x). |
Source: Buenaventura Ingenieros S.A. (2014).
Flotation Test of the Cleaning Circuit Using Denver Cells
Metallurgical tests were conducted to enhance the recovery and quality of zinc concentrate based on its mineralogical characterization. Third cleaner flotation tests were carried out using the specific parameters listed in Table 2.
Additionally, standard cleaner flotation tests were carried out, and the metallurgical balance of the most representative test is displayed in Table 3. The effectiveness of cuprocyanide as a secondary polymetallic reagent was tested at varying dosages of 25, 30, 35, 45, and 50 g/t, in combination with other reagents including lime, dextrin, and quebracho. The metallurgical balances of the different tests are shown in Tables 4, 5, 6, 7, and 8.
Representative samples of the froth from the second cleaner flotation were taken under normal conditions, with an average zinc grade of 51.5%. The purpose was to determine which test yielded the best flotation results and performance in the zinc cleaning circuit section. The metallurgical factor was compared to achieve this (Manzaneda, 2010).
The operating conditions used to conduct the metallurgical tests are shown in Table 2.
Table 2. Operating Conditions for Flotation Tests.
|
Variable |
Value |
|
pH |
8.25 |
|
Solids % |
33.01 |
|
Particle Size Distribution |
68.2% - 200 m |
|
Density |
1.18 kg/L |
|
Specific Gravity |
2.20 |
|
Flotation Period |
2 min |
|
Conditioning Time |
2 min |
|
Reagent |
Cuprocyanide |
Source: Prepared by the authors.
Table 3. Metallurgical Balance for Standard Cleaner Flotation Test.
|
Product |
Weight (%) |
Grades |
Recovery |
||||||||
|
Ag oz/t |
Pb% |
Zn% |
Cu% |
Fe% |
Ag oz/TM |
Pb% |
Zn% |
Cu% |
Fe% |
||
|
Zn Concentrate |
66.01 |
2.45 |
0.74 |
51.78 |
0.59 |
9.24 |
41.94 |
37.02 |
72.66 |
45.93 |
63.42 |
|
Cleaner Tail |
33.99 |
6.58 |
2.43 |
37.84 |
1.35 |
10.35 |
58.06 |
62.98 |
27.34 |
54.07 |
36.58 |
|
Head Calculated |
100.00 |
3.85 |
1.31 |
47.04 |
0.85 |
9.62 |
100.00 |
100.00 |
100.00 |
100.00 |
100.00 |
Source: Prepared by the authors.
Table 4. Metallurgical Balance for Cleaner Flotation Test Using 25 g/t Cuprocyanide.
|
Product |
Weight (%) |
Grades |
Recovery |
||||||||
|
Ag oz/t |
Pb% |
Zn% |
Cu% |
Fe% |
Ag oz/TM |
Pb% |
Zn% |
Cu% |
Fe% |
||
|
Zn Concentrate |
58.60 |
2.53 |
0.70 |
52.63 |
0.56 |
8.46 |
38.49 |
31.55 |
68.40 |
36.54 |
52.66 |
|
Cleaner Tail |
41.40 |
5.72 |
2.15 |
34.42 |
1.36 |
10.76 |
61.51 |
68.44 |
31.60 |
63.45 |
47.34 |
|
Head Calculated |
100.00 |
3.85 |
1.30 |
45.09 |
0.89 |
9.41 |
100.00 |
100.00 |
100.00 |
100.00 |
100.00 |
Source: Prepared by the authors.
Table 5. Metallurgical Balance for Cleaner Flotation Test Using 30 g/t Cuprocyanide.
|
Product |
Weight (%) |
Grades |
Recovery |
||||||||
|
Ag oz/t |
Pb% |
Zn% |
Cu% |
Fe% |
Ag oz/TM |
Pb% |
Zn% |
Cu% |
Fe% |
||
|
Zn Concentrate |
56.51 |
2.45 |
0.69 |
53.15 |
0.57 |
8.33 |
36.00 |
31.25 |
64.06 |
36.62 |
50.00 |
|
Cleaner Tail |
43.49 |
5.66 |
1.96 |
38.75 |
1.29 |
10.83 |
64.00 |
68.75 |
35.94 |
63.38 |
50.00 |
|
Head Calculated |
100.00 |
3.85 |
1.24 |
46.89 |
0.89 |
9.42 |
100.00 |
100.00 |
100.00 |
100.00 |
100.00 |
Source: Prepared by the authors.
Table 6. Metallurgical Balance for Cleaner Flotation Test Using 35 g/t Cuprocyanide.
|
Product |
Weight (%) |
Grades |
Recovery |
||||||||
|
Ag oz/t |
Pb% |
Zn% |
Cu% |
Fe% |
Ag oz/TM |
Pb% |
Zn% |
Cu% |
Fe% |
||
|
Zn Concentrate |
66.44 |
2.54 |
0.72 |
52.24 |
0.60 |
0.68 |
44.14 |
38.52 |
73.80 |
46.38 |
61.87 |
|
Cleaner Tail |
33.56 |
6.37 |
2.28 |
36.72 |
1.37 |
10.59 |
55.86 |
61.48 |
26.20 |
53.62 |
38.13 |
|
Head Calculated |
100.00 |
3.83 |
1.25 |
47.03 |
0.86 |
9.32 |
100.00 |
100.00 |
100.00 |
100.00 |
100.00 |
Source: Prepared by the authors.
Table 7. Metallurgical Balance for Cleaner Flotation Test Using 45 g/t Cuprocyanide.
|
Product |
Weight (%) |
Grades |
Recovery |
||||||||
|
Ag oz/t |
Pb% |
Zn% |
Cu% |
Fe% |
Ag oz/TM |
Pb% |
Zn% |
Cu% |
Fe% |
||
|
Zn Concentrate |
66.44 |
2.47 |
0.72 |
52.52 |
0.60 |
8.45 |
41.24 |
35.82 |
72.00 |
44.61 |
58.09 |
|
Cleaner Tail |
33.56 |
6.39 |
2.33 |
37.01 |
1.36 |
11.04 |
58.76 |
64.18 |
28.00 |
55.39 |
41.91 |
|
Head Calculated |
100.00 |
3.86 |
1.29 |
47.00 |
0.87 |
9.37 |
100.00 |
100.00 |
100.00 |
100.00 |
100.00 |
Source: Prepared by the authors.
Table 8. Metallurgical Balance for Cleaner Flotation Test Using 50 g/t Cuprocyanide.
|
Product |
Weight (%) |
Grades |
Recovery |
||||||||
|
Ag Oz/t |
Pb% |
Zn% |
Cu% |
Fe% |
Ag oz/TM |
Pb% |
Zn% |
Cu% |
Fe% |
||
|
Zn Concentrate |
46.31 |
2.27 |
0.64 |
53.78 |
0.56 |
8.13 |
27.96 |
24.98 |
52.94 |
29.64 |
40.88 |
|
Cleaner Tail |
53.69 |
5.04 |
1.73 |
41.24 |
1.15 |
10.15 |
72.04 |
75.72 |
47.06 |
70.36 |
59.12 |
|
Head Calculated |
100.00 |
3.76 |
1.23 |
47.05 |
0.88 |
9.21 |
100.00 |
100.00 |
100.00 |
100.00 |
100.00 |
Source: Prepared by the authors.
Figure 5 displays a bar chart comparing the percentage of zinc grade, recovery, and metallurgical factor of the various tests shown above. Additionally, Figure 6 shows a graph of the cuprocyanide evaluation in the third cleaner flotation of the zinc circuit.
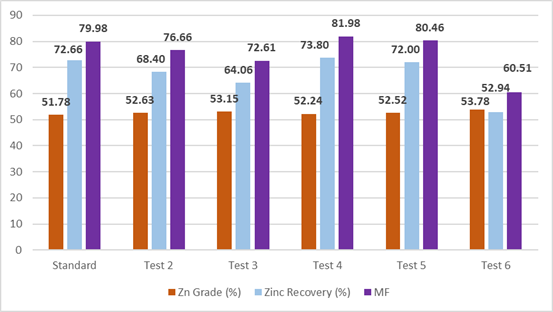
Figure 5. Bar chart comparing standard flotation test vs. flotation tests using cuprocyanide.
Source: Prepared by the authors.
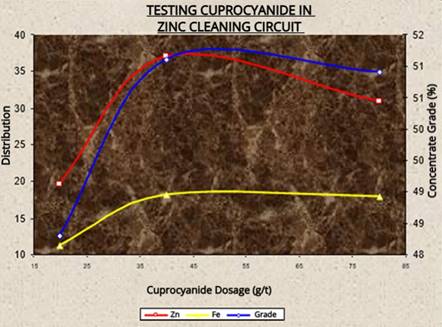
Figure 6. Kinetics of cuprocyanide during the cleaner flotation process of the zinc circuit.
Source: Prepared by the authors.
Hypothesis Testing
Table 3 displays the results of the standard cleaner flotation process, which recovers 72.66% zinc with a grade of 51.78%. Similarly, Table 6 shows the results of flotation using 35 g/t cuprocyanide, which recovers 73.80% zinc with a grade of 52.24%. These results validate the hypothesis of the research.
DISCUSSION
The mineralogical characterization conducted revealed the presence of type 2 sphalerite, with a particle size range of 35-150 μm. This finding is reflected in the metallurgical balances of the various samples, which show a minimum copper displacement ranging between 0.56% and 0.60% in the zinc concentrate.
The laboratory tests revealed that the amount of secondary collector reagent cuprocyanide can regulate the recoveries and grade of the zinc concentrates. The standard cleaner flotation test recovered 72.66% zinc concentrate with a grade of 51.78% and a metallurgical factor of 79.98%. This was used as a baseline for comparison with the subsequent tests using different dosages of the cuprocyanide reagent.
The flotation cleaner tests using different cuprocyanide reagent dosages revealed that recovery percentage, grade, and metallurgical factor vary depending on the dosage of the reagent used. At a dosage of 35 g/t cuprocyanide, a recovery of 73.80% was achieved with a grade of 52.24% and a metallurgical factor of 81.98, thus reporting the lowest Zn grade value. Meanwhile, a dosage of 50 g/t recovered 52.94% zinc concentrate with a grade of 53.78%, and a metallurgical factor of 60.50, thus reporting the highest Zn grade value in the concentrate.
It can be observed from Figure 6 that the quality and recovery percentage improve as the cuprocyanide dosage increases. However, the trend changes to a negative slope when the dosage exceeds a certain threshold. The optimal dosage is around 35 g/t, and the maximum dosage limit is 55 g/t.
Our research bears some similarity to the one conducted by Xu et al. (2022) in terms of the mineralogical composition of the samples analyzed, which mainly consisted of sulfides such as sphalerite, chalcopyrite, and pyrite. However, there is a significant difference in the methodologies used for the mineralogical analysis between the two research studies. Scanning microscopy and X-ray diffraction were found to be more detailed and accurate than reflected light optical microscopy.
Additionally, in their research, Xu et al. (2022) were able to achieve a zinc grade of 45.97% using butyl xanthate (BX) as a collector with dosages of 80 g/t in the rougher stage in their most representative test. However, there was a difference of 7.81% in the concentrate grade when compared to our research. The reason behind this difference could be attributed to the fact that they did not use reagents in the cleaner stage.
The main difference between the study conducted by Palafox et al. (2010) and this research lies in the use of statistical methods to optimize the relevant parameters for the sale of zinc concentrate, recovery, and grade. Their research achieved maximum values of 96.7% and 57.0%, respectively, which are higher than those achieved in our research. Furthermore, the authors took into account particle size and gas dispersion in the flotation cells when optimizing the process.
CONCLUSIONS
- The mineralogical analysis revealed that type 1 and type 2 sphalerite constitute 6.29% and 0.40% of the volume, respectively. The grades of liberation for type 1 and type 2 are 87.41% and 71.43%, respectively.
- The lowest zinc concentrate grade was 52.24%, obtained by dosing 35 g/t of cuprocyanide, while the highest grade was 53.78%, obtained by dosing 50 g/t of the reagent.
- In terms of zinc recovery percentage, the lowest value was 52.64%, obtained by dosing 50 g/t of cuprocyanide, while the highest value was 73.80%, obtained by dosing 35 g/t of the reagent.
- The third cleaner flotation metallurgical test, which used 35 g/t of cuprocyanide, yielded a metallurgical factor of 81.98 and was deemed the most representative.
- The results from the zinc 3rd cleaner flotation tests indicate that the minimum silver content is 2.27 oz/MT when using 50 g/t cuprocyanide, while a silver grade of 2.45 oz/MT can be achieved with 35 g/t. Interestingly, the reagent dosage does not seem to affect the silver content in the concentrate. The presence of silver in the zinc concentrate may be due to the displacement of galena, which is associated with this precious metal.
- Lead in the zinc concentrate has a displacement that varies between 0.64% and 0.74%.
- Copper in the zinc concentrate has a displacement that varies between 0.56% and 0.60%.
REFERENCES
[1] Arias Gonzales, J. L., & Covinos Gallardo, M. (2021). Diseño y metodología de la investigación (1st ed.). Arequipa, Peru: Enfoques Consulting EIRL.
[2] Azañero Ortiz, A., Aramburu Rojas, V., Quiñones Lavado, J., Puente Santibáñez, L., Cabrera Sandoval, M., Rengifo Sing, W., Falconi Rosadio, V., & Quispe Valdivia, J. (2010). Flotación de minerales polimetálicos sulfurados de Pb, Cu y Zn. Revista del Instituto de investigación de la facultad de Minas, Metalurgia y Ciencias Geográficas, 13(26), 51-58. https://revistasinvestigacion.unmsm.edu.pe/index.php/iigeo/article/view/429
[3] Buenaventura Ingenieros S.A. (2014). Análisis mineralógicos por microscopía óptica y estudios por microscopía electrónica de ocho muestras tamizadas (Ensayo de informe: IL-003ES0014LB-000-50-0097). BISA.
[4] Cortez Marcelo, C. (2019). Flotación de zinc sin el uso de cal para la recuperación de concentrado de zinc, en la Unidad deProducción Andaychagua - Compañía Minera Volcan S.A.A.- 2019. (Degree thesis). Universidad Nacional Daniel Alcides Carrión, Cerro de Pasco. http://repositorio.undac.edu.pe/bitstream/undac/1671/1/T026_47674022_T.pdf
[5] Inga Paucar, A. (2020). Optimización en la recuperación de zinc de minerales polimetálicos mediante el proceso de flotación en la empresa Mines and Metals Trading Perú - Huancavelica. (Degree thesis). Universidad Nacional Mayor de San Marcos, Lima. https://cybertesis.unmsm.edu.pe/item/8dbe9424-6853-4996-9bbc-9aa042173ec4
[6] Inga Paucar, A., Aramburú Rojas, V. S., & Tiburcio Alva, R. M. (2020). Optimización en la Recuperación de Zinc de minerales polimetálicos mediante el proceso de flotación en la empresa Mines and Metals Trading Perú - Huancavelica. Industrial Data, 23(2), 21-30. https://doi.org/10.15381/idata.v23i2.16632
[7] Instituto Peruano de Economía. (2022). Índice de Competitividad Regional - INCORE 2022. https://incoreperu.pe/portal/images/financepress/ediciones/INCORE_2022.pdf
[8] Kyaw, Z., Tiagalieva, Z., Htet, Z., & Phyo, K. (2021). Improvement of reagent flotation modes of sphalerite and pyrite from deposits of copper-zinc pyrite, polymetallic copper-zinc pyrite and polymetallic ores. IOP Conference Series: Earth and Environmental Science, 942. https://doi.org/10.1088/1755-1315/942/1/012004
[9] Li, J., Song, K., Zhang, X., Li, J., & Liu, D. (2017). Effect of Combined Reagents of Sodium Citrate and Sodium Pyrophosphate on Flotation Separation of a Polymetallic Lead-Zinc Ore. The Chinese Journal of Process Engineering, 17(3), 500-505. https://doi.org/10.12034/j.issn.1009-606X.216316
[10] Li, B., Xu, M., Gong, C., & Li, P. (2017). Hotspots and Trends in International Soil Quality Research. Journal of Natural Resources, 32(11), 1983-1998. https://doi.org/10.11849/zrzyxb.20160968
[11] Manzaneda Cabala, J. R. (2010). Aplicación de microscopía en el procesamiento de minerales por flotación. (Master's thesis). Universidad Nacional de Ingeniería, Lima. http://hdl.handle.net/20.500.14076/611
[12] Mateos, M. (2020). Efecto de la salinidad en la flotación de esfalerita y marmatita. (Master's thesis). Universidad Michoacana de San Nicolás de Hidalgo, Morelia.
[13] Ministerio de Energía y Minas. (2021). Boletín Estadístico Minero (Edición N° 12-2021). https://www.minem.gob.pe/minem/archivos/file/Mineria/PUBLICACIONES/VARIABLES/2021/BEM12-2021.pdf
[14] Natarajan, R., & Nirdosh, I. (2006). New collectors for sphalerite flotation. International Journal of Mineral Processing, 79(3), 141-148. https://doi.org/https://doi.org/10.1016/j.minpro.2005.11.011
[15] Palafox Méndez, C., De la Fuente Zamarripa, D., Castillo Mendoza, J., & Reyes Bahena, J. L. (2010). Rediseño del circuito de flotación de zinc usando modelación matemática. XV Encuentro sobre Procesamiento de Minerales, 1-14.
[16] Tang, H., Deng, Z., Tang, Y., Tong, X., & Wei, Z. (2023). Hotspots and trends of sphalerite flotation: Bibliometric analysis. Separation and Purification Technology, 312(123316). https://doi.org/10.1016/j.seppur.2023.123316
[17] U.S. Geological Survey. (2022). Mineral Commodity Summaries 2022. https://doi.org/10.3133/mcs2022
[18] Utepbaeva, S., Urazbayeva, S., Joldasbaeva, J., & O’telbayev, A. (2023). Foam flotation process, stages and technological parameters. Science and Innovation, 2(2), 136-140. https://doi.org/10.5281/zenodo.7641035
[19] Xu, B., Zhong, S, Wu, J., Zhou, Y., Yang, Y., Li, Q., & Jiang, T. (2022). A Comprehensive Recovery Process for Selective Separation and Enrichment of Copper, Zinc and Iron Minerals from a Polymetallic Ore and the Adsorption Mechanism of Collector Z-200. Minerals, 12(3). https://doi.org/10.3390/min12030384
[20] Yu, J., Wu, X., Zhao, Z., Zhu, Y., & Luo, S. (2019). Effect of a Small Amount of Iron Impurity in Sphalerite on Xanthate Adsorption and Flotation Behavior. Minerals, 9(11), 687. https://doi.org/10.3390/min9110687