Revista Industrial Data 27(2): 7-31 (2024)
DOI: https://doi.org/10.15381/idata.v27i2.23859
ISSN: 1560-9146 (Impreso) / ISSN: 1810-9993 (Electrónico)
Optimizing Pb-Ag Recovery Rate in the
Flotation Process of Polymetallic Ores from the Northern Andean Region of Peru through
Mineralogical Characterization
Design and
Technology
Submitted:
26/10/2022 Accepted: 13/09/2023 Published: 18/12/2024
https://doi.org/10.15381/idata.v27i2.23859.g204481
José David Valverde Díaz
Vidal Sixto Aramburú Rojas
Jorge Alberto Ortiz Barreto
Sharon Elisa Aguilar Zevallos
ABSTRACT
A mineralogical characterization study to optimize
lead-silver (Pb-Ag) recovery rate by flotation was conducted at three adits named
Esmeralda, Orión, and Hércules. The variables investigated included the dosage
of the reagent blend (AP-3418/AR-404), AR-242, and pH. At the Esmeralda adit, a
recovery of 81.99% lead at a concentrate grade of 58.66% and 83.79% silver at a
head grade of 310.64 oz/t was achieved using a dosage of 18 g/t reagent blend
(AP-3418/AR-404), 5 g/t AR-242, and an optimum pH value of 11.5. At the Orión adit,
a recovery of 81.71% lead at a concentrate grade of 61.6%, and 81.24% silver at
a head grade of 85.39 oz/t were achieved using a dosage of 30 g/t reagent blend
(AP-3418/AR-404), 5 g/t AR-242, and an optimum pH value of 10.5. Finally, at
the Hércules adit, a recovery of 82.4% lead at a concentrate grade of 54.12%,
and 80.88% silver at a head grade of 62.63 oz/t grade were achieved using a
dosage of 30 g/t reagent blend (AP-3418/AR-404), 15 g/t AR-242, and a pH value
of 11.5.
Keywords:
characterization, Minitab, adit, flotation.
INTRODUCTION
Until the end of 2019, Peru ranked as the second-largest silver producer
and the third-largest lead producer in the world, according to a report by the
United States Geological Survey (2019). However, from 2020 onwards, there has
been a decline in the production of these metals. As a result, Peru has dropped
to the third and fourth positions for silver and lead production worldwide,
respectively, surpassed by China and the United States, according to figures
reported by the Peruvian Statistical Mining Bulletin (Ministerio de Energía y
Minas, 2022).
The lead content of natural galena is generally low, requiring
concentration to obtain a commercial product (Yekeler & Yekeler, 2006).
Flotation has been the conventional method for recovering polymetallic ores,
with low production costs and low environmental impact. As a result, it remains
irreplaceable in the processing of sulfide ore resources (Xie et al., 2021).
Notably, about 95% of global lead production comes from its sulfide species
(Zou et al., 2022). The success of the flotation process can be attributed to
the ability of the chemical reagents to form stable complexes with metal ions
in aqueous solutions or on ore surfaces. This enhances the physical and
chemical properties of the surface, allowing for the selective separation of
sulfide species from the gangue (Chen, 2021). Dithiophosphoric acid compounds
have been developed as flotation collectors for the sulfide species of lead,
among them, dithiophosphate (DTP) and dithiophosphinate (DTPI) are the most
widely used due to their high selectivity in galena concentration (Tercero et
al., 2019). Concentrating galena with xanthate-type collectors is also
possible, which do not hydrolyze, oxidize, or decompose into other species,
unlike dithiophosphates (Elizondo et al., 2021; Shen et al., 2016).
Silver is often found as a trace or minor component in galena; as a
result, less attention is often given to silver recovery compared to the main
component (Ayllón, 2013;
Aranda, 2014; Nassar et al., 2015; Song et al., 2021). However, the
significance of silver has grown due to its role in the development of green
technologies, such as solar panels and hybrid vehicles. This has made
optimizing silver production during the flotation process critical for the
mining and metallurgical sectors (Tiu et al., 2021). Furthermore, silver is
recognized as one of the essential materials needed to achieve the Sustainable
Development Goals (SDGs) set for 2030 (Dou et al., 2023).
Astucuri (1994)
discusses the intricate physical and chemical characteristics of certain
species, emphasizing the need for a thorough geological characterization of the
deposits that contain them. This process involves identifying and quantifying
mineral species, their volumetric distribution, degrees of liberation, and the
associations in the ore; it also includes analyzing rock structures associated
with the deposit (Yovanovic, 2004; Melgarejo et al., 2010; Bertolino et al.,
2014; Ramos & Orihuela, 2017; Taya, 2018). Such detailed information is
crucial for assessing the impact of the species involved in mineral processing;
it aids in effectively segmenting the deposit and optimizing the recovery of
valuable species (López & Ipanaqué, 2008; Bustamante et al., 2008; Ojeda et
al., 2010; Espinoza et al., 2021).
Among the conventional techniques used for the characterization of
mineral species are X-ray powder diffraction (XRD), quantitative X-ray
diffraction, scanning electron microscopy and energy dispersive X-ray
spectroscopy (SEM-EDS), cathodoluminescence, and electron microprobe (EMP).
These are widely used due to their relatively low cost. In contrast,
non-conventional techniques such as particle-induced X-ray emission
(Micro-PIXE), secondary ion mass spectrometry (SIMS), and laser ablation
inductively coupled plasma mass spectrometry (LA-ICP-MS) are less accessible
and significantly more expensive (Melgarejo et al., 2010; Alves, 2014).
Song et al. (2021) investigated
the flotation of silver-bearing galena using collectors such as dibutyl
ammonium dithiophosphate (ADD), ethyl xanthate (EX), and diethyldithiocarbamate
(DDC). They found that silver-bearing galena is more effectively floated at a
pH level of 9.5. The results indicated that the ADD collector yielded better
recovery rates than both the EX and DDC collectors. The authors conducted micro-flotation
experiments on 2 g samples ground to 100% −200 mesh. They also performed
conventional flotation tests in a 1500 ml flotation cell using 500 g of raw ore
ground to 80% −74 microns, an agitation rate of 1500 rpm, and an airflow rate
of 20 L/min. To further investigate the behavior of silver-bearing galena, the
authors conducted simulations based on the density functional theory (DFT)
method. The best flotation tests using the ADD collector achieved a recovery
rate of 85% for galena and over 90% for silver-bearing galena.
This research paper is relevant because it uses experimental designs and
analysis of variance to determine the optimal dosage of reagents for maximizing
the recovery rate and quality of Pb-Ag concentrate. The calculated reagent
dosages aim to predict recovery percentages and quality in polymetallic
deposits located in the northern Andean region of Peru. Additionally, this
research contributes to the improvement and optimization of outcomes in Pb and
Ag mining, while also enhancing the academic understanding related to their
extraction.
Optimizing lead-silver recovery rate in the
flotation process of polymetallic ores from the Andean region of Peru through mineralogical
characterization and experimental designs.
Specific
Objectives
· Identify the sample components, degrees of
liberation, and the presence of free and interlocked grains from the Esmeralda,
Orión, and Hércules adits in the Andean region of Peru.
·
Determine the influence
of the reagent blend (AP-3418/AR-404) dosage, AR-242, and the pH of the pulp,
along with their contributions to the flotation process using Plackett-Burman
experimental designs and Minitab statistical software.
·
Optimize the lead and
silver recovery rate in the flotation process of polymetallic ores from three adits
located in the Andean region of Peru.
General Hypothesis
Mineralogical characterization and experimental designs optimize the lead-silver
recovery rate by the flotation process of polymetallic ores in the Andean region
of Peru.
Specific
Hypotheses
·
Mineralogical
characterization allows for the identification of the mineral species present
in a sample, as well as the degrees of liberation and bonds found in various
mineralogical formations.
·
Analyzing the
interaction of reagent blend (AP-3418/AR-404) dosages, AR-242, and the pH of
the pulp using Plackett-Burman experimental designs in Minitab statistical
software helps determine the extent of their contribution to the flotation
process in various adits.
·
Optimizing the lead and
silver recovery rate by flotation leads to improved metallurgical performance.
Theoretical Justification
The optimization of reagent dosage and pulp alkalinity in the flotation
process enhances the metallurgical performance of lead-silver
concentrates, thereby improving concentrate quality and resulting in a positive
economic impact.
Practical Justification
The identification of species is essential for characterizing each
mineralogical formation involved in the concentration process. This
understanding is important to optimize the recovery rate of the concentrate
through flotation of polymetallic ores, considering reagent dosage and pulp
alkalinity variables, in the Andean regions of Peru. Furthermore, the findings
of this study can serve as a model for other mines with similar mineralogy,
improving lead and silver recovery using experimental designs in flotation.
Economic Justification
Effective management of raw materials is essential for optimizing the
production of metallurgical mining resources, which can significantly enhance
the revenue of a concentrator plant.
Social Justification
From a social perspective, optimizing resource use in the processing of
polymetallic ores through the flotation process benefits both directly and
indirectly involved communities. Thus, this approach helps guarantee local
employment and fosters community development for future generations.
METHODOLOGY
This research employs a quantitative, deductive, and experimental design
methodology. It applies knowledge of mineralogical characterization and
analyzes the variables influencing the flotation process, which facilitates the
optimization of the lead-silver concentrate recovery rate. The research
follows a specific design framework as shown in Figure 1.
Figure 1. Methodological
scheme of the research.
Note. The figure
illustrates the measurements included in the research design for this study.
Source: Prepared by the authors.
O1: Mineralogical characterization of the Esmeralda, Orión,
and Hércules adits.
X: Development of the Plackett-Burman
experimental design and analysis of the variables influencing the metallurgical
flotation process.
O2: Optimization of lead and silver recovery in the high
Andean region of Peru.
Research Method Procedure
·
Sampling and Sample Preparation
A representative sample of polymetallic ore was
collected from the Esmeralda, Orión, and Hércules adits. Using a jaw crusher, this
sample was then ground to 100% passing through a 10 mesh. To ensure thorough
research testing, the entire sampled batch was quartered, and a portion was
stored as a control sample.
·
Chemical Assays and
Mineralogical Characterization
A representative ore sample was ground in a ball mill
to determine the head grades of lead (Pb) and silver (Ag). Additionally,
samples of different gradation (+100 mesh, <−100, +200] mesh, and <−200,
+400] mesh) from the study adits were sent to the mineralogical
characterization laboratory to identify the mineral species present, assess
volumetric distribution, determine degrees of liberation, and evaluate associations
or bonds present. The mineralogical analysis was performed using a polarized
light microscope.
·
Experimental Designs Using Minitab
19
Plackett-Burman experimental designs were generated
using Minitab 19 statistical software to assess the independent variables that
significantly influence the recovery and quality of lead-silver
concentrate, as well as to optimize the flotation process. A test plan was
established based on the Plackett-Burman template provided by the software to
assess the impact of the reagent blend (AP-3418/AR-404, AR-242) and the pH of
the pulp on the recovery and quality of the lead-silver concentrate. Other
process variables were kept constant during the laboratory metallurgical
flotation tests.
RESULTS
Chemical Assays
The chemical assays for lead (Pb) in the study adits were conducted using
the volumetric analysis, while silver (Ag) head grades were determined through
cupellation. Laboratory reports indicated that the Esmeralda adit exhibited the
highest silver head grade (6.65 oz/t), whereas the Hércules adit showed the
lowest silver head grade (1.12 oz/t). Additionally, the reports on lead
concentration revealed that the Orión adit had the highest lead head grade (1.46%).
The results of the chemical assays conducted in the study adits are summarized
in Table 1 below.
Table 1. Chemical Assays Performed on Samples from the Adits.
|
Adit
|
Ag head grade
(oz/t)
|
Pb head grade
(%)
|
|
Esmeralda
|
6.65
|
1.22
|
|
Orión
|
1.59
|
1.46
|
|
Hércules
|
1.12
|
0.89
|
Note. The table displays the Ag-Pb head grades for the
Esmeralda, Orión, and Hércules adits.
Source: Prepared by the authors.
Volumetric Distribution of the Esmeralda, Orión, and Hércules Adits
The analysis of a representative sample from the Esmeralda adit, observed
under a polarizing light microscope, revealed that the primary lead-bearing
minerals are galena and lead sulfosalts. These minerals were predominantly
found in the particle size range of (−74 µ + 37 µ). Contaminating minerals such
as arsenopyrite were identified, with a significant distribution of 3.62%. Sphalerite
I and II were also identified, showing distribution values of 2.4% and 0.48%,
respectively. The presence of fahlores was noted with a distribution of 0.19%,
and stibnite was found with a distribution of 0.33%, all achieved at the
particle size distribution range of (−74 µ + 37 µ).
The mineralogical characterization of a representative sample from the
Orión adit determined that galena is the main lead-bearing species. The highest
distribution of this species occurs at a particle size smaller than 74 µ, with
a reported value of 1.02%. Contaminant ores, such as arsenopyrite, were also identified,
showing the highest distribution of 3.81% at a particle size smaller than 200
mesh. Sphalerite I and II were also identified, showing distribution values of
2.61% and 0.35%, respectively, at a particle size smaller than 200 mesh. The
presence of fahlores was noted with a maximum value of 0.08% retained on 200
mesh, and stibnite was found with a distribution of 0.41% achieved at a
particle size distribution range of 100% −200 mesh.
The analysis conducted for the Hércules adit revealed that galena is the
primary lead-bearing mineral, with a maximum distribution value of 2.21% at a particle
size smaller than 200 mesh. Arsenopyrite was also detected with a maximum
distribution of 4.95% at particles larger than 74 µ, while type I sphalerite
exhibited a maximum distribution of 13.25% at particles smaller than 74 µ.
Notably, the volumetric distribution of type I sphalerite was the highest
compared to other adits. It is important to mention that fahlores, type II sphalerite,
and stibnite were not reported.
The volumetric distribution values of all minerals identified under the
reflected light optical microscope are shown in Table 2, categorized by gradation:
+100 mesh, <−100, +200] mesh, and <−200, +400] mesh. The data shows that
these minerals are found in greater volumes at finer particle sizes, suggesting
that the species are liberated in this range of particle size distribution.
Degree of Liberation of Minerals from Esmeralda, Orión, and Hércules Adits
The mineral liberation analysis conducted at Esmeralda adit to determine
the degree of liberation of galena and sulfosalts reported values of 86.08% and
78.82%, respectively, at a particle size of 100% −200 mesh. Regarding
contaminants, arsenopyrite exhibited a degree of liberation of 95.06%; fahlores
showed a degree of liberation of 76.88%; sphalerite revealed a degree of
liberation of 100%; and stibnite showed a degree of liberation of 91.66%. These
values were determined at a particle size smaller than 200 mesh. Additionally,
the highest degree of liberation for type I sphalerite was found at a particle
size of 100% −100 mesh.
The mineral liberation analysis conducted at Orión adit revealed that
galena exhibited a degree of liberation value of 91.72% at a particle size
smaller than 200 mesh. At this same particle size distribution, the maximum
degrees of liberation were found for type I sphalerite, arsenopyrite, and
stibnite, with values of 95.2%, 95.11%, and 97.63%, respectively. Notably, type
II sphalerite exhibited the highest degree of liberation of 93.55% for material
retained on 100 mesh, while fahlores showed a value of 88.9% for material
retained on 200 mesh.
The mineral liberation analysis conducted at Hércules adit revealed that
galena was found to be 97.62% liberated at a particle size of 100% −200 mesh.
In contrast, the minerals considered detrimental to the process such as
sphalerite I, sphalerite II, and arsenopyrite reported liberation values of
96.14%, 44.89%, and 97.38%, respectively, at a particle size range of <−100,
+200] mesh. The total degrees of liberation for the identified species at
various gradations are summarized in Table 3.
Types of bonding between Pb and Ag
A representative sample from the Esmeralda adit was analyzed to determine
the bonds present. Considering a particle size of 100% +100 mesh, galena was
found to form bonds with pyrite, gangue, fahlores, and type I sphalerite, all
of which were reported to be moderately easy to liberate. At a particle size of
100% −100mesh, galena formed bonds
with arsenopyrite, pyrite, gangue, and fahlores. The bonds formed with gangue,
pyrite, and arsenopyrite were reported to be very difficult to liberate. At a
particle size of 100% −200 mesh, galena formed bonds with fahlores, lead
sulfosalts, type I sphalerite, arsenopyrite, stibnite, and chalcopyrite. Among
these, the bonds with fahlores were described as moderately difficult to
liberate, while those with lead sulfosalts were classified as very difficult.
Similarly, a representative sample from the Orión adit was also analyzed
to determine the bonds present. Considering a particle size of 100% +100 mesh,
galena formed bonds with arsenopyrite, which was found to be very difficult to
liberate. At a particle size of 100% −100 mesh, the bonds formed between galena
and chalcopyrite were deemed very difficult to liberate. Finally, at a particle
size of 100% −200 mesh, the bond formed between galena and arsenopyrite was considered
impossible to liberate.
In contrast, the bonds formed between galena and other minerals found in
a representative sample from the Hércules adit were reported to be easy to
liberate.
A comprehensive description of the observed bonds and their liberation
potential for the various samples is detailed in Table 4. Additionally, Figures
2, 3, and 4 depict the main micrographs of galena from the study adits.
Table 4. Types Of Bonding at Various Particle Size Distribution
Ranges
for the Esmeralda, Orión, and Hércules Adits.
|
Particle size
|
Esmeralda Adit
|
Orión Adit
|
Hércules Adit
|
|
Bonding
|
Vol. (%)
|
Liberation potential
|
Bonding
|
Vol.
(%)
|
Liberation potential
|
Bonding
|
Vol.
(%)
|
Liberation potential
|
|
+100 mesh
|
py/gn
|
0.04
|
Moderately easy
|
gn/sph I
|
0.09
|
Easy
|
|
|
|
|
GGs/gn
|
0.08
|
Moderately easy
|
GGs/gn
|
0.23
|
Very difficult
|
|
|
|
|
GGs/py/gn
|
0.25
|
Moderately difficult
|
apy/gn
|
0.19
|
Very difficult
|
py/gn
|
1.79
|
Easy
|
|
GGs/gn/Fhls
|
0.08
|
Moderately easy
|
py/gn/sph I
|
0.09
|
Moderately easy
|
gn/sph I
|
0.83
|
Moderately easy
|
|
GGs/py/gn/sph I
|
0.08
|
Moderately easy
|
GGs/apy/gn
|
0.09
|
Moderately easy
|
|
|
|
|
|
|
|
GGs/gn/sph I
|
0.19
|
Moderately easy
|
|
|
|
|
<−100, +200] mesh
|
apy/gn
|
0.08
|
Easy
|
gn/sph I
|
0.24
|
Easy
|
|
|
|
|
py/gn
|
0.08
|
Easy
|
apy/gn
|
0.08
|
Easy
|
|
|
|
|
GGs/gn
|
0.08
|
Very difficult to impossible
|
cp/gn
|
0.08
|
Very difficult to impossible
|
gn/sph I,
|
0.02
|
Easy
|
|
gn/Fhls
|
2.00
|
Moderately easy
|
GGs/gn
|
0.16
|
Moderately easy
|
gn/sph II
|
0.16
|
Easy
|
|
GGs/py/gn
|
0.08
|
Moderately difficult
|
GGs/cp/CGRs
|
0.08
|
Moderately easy
|
|
|
|
|
py/apy/gn
|
1.00
|
Very difficult
|
|
|
|
|
|
|
|
<−200, +400] mesh
|
gn/Fhls
|
0.10
|
Moderately difficult
|
|
|
|
|
|
|
|
gn/Pb sulfosalts
|
0.29
|
Very difficult
|
|
|
|
|
|
|
|
gn/sph I
|
0.10
|
Easy
|
gn/sph I
|
0.12
|
Moderately easy
|
sph I/gn
|
0.11
|
Moderately easy
|
|
apy/gn
|
0.19
|
Moderately easy
|
py/gn
|
0.12
|
Moderately easy
|
GGs/apy/gn
|
0.17
|
Moderately easy
|
|
gn/stb
|
0.10
|
Easy
|
apy/gn
|
0.23
|
Impossible
|
|
|
|
|
cp/gn/CGRs
|
0.10
|
Moderately easy
|
|
|
|
|
|
|
|
py/gn/CGRs
|
0.10
|
Moderately easy
|
|
|
|
|
|
|
Note. The table shows the galena forming bonds with other ores
and their liberation potential.
Source: Prepared by the authors.
|
MAIN MICROGRAPHS OF GALENA IN THE STUDY ADITS
|
|
ESMERALDA
|
ORIÓN
|
HÉRCULES
|
|
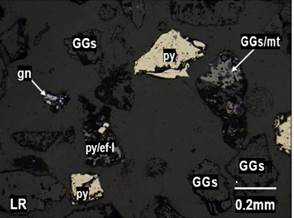 Figure 2. Liberated grains of gangues (GGs), pyrite (py), galena (gn), mixed
gangue/magnetite (GGs/mt), and pyrite/sphalerite I (py/sphI) found in
particle sizes of +100 mesh. Figure 2. Liberated grains of gangues (GGs), pyrite (py), galena (gn), mixed
gangue/magnetite (GGs/mt), and pyrite/sphalerite I (py/sphI) found in
particle sizes of +100 mesh.
|
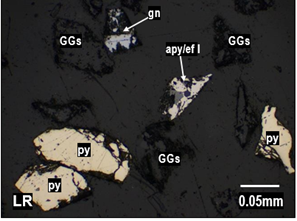 Figure 3. Liberated grains of gangues
(GGs), pyrite (py), galena (gn), and arsenopyrite/sphalerite I (apy/sph I) found
in the particle size distribution range of Figure 3. Liberated grains of gangues
(GGs), pyrite (py), galena (gn), and arsenopyrite/sphalerite I (apy/sph I) found
in the particle size distribution range of
<−100, +200] mesh.
|
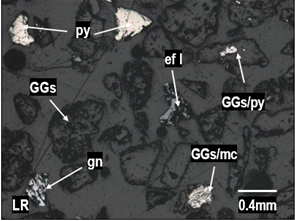 Figure 4. Liberated grains of pyrite (py),
sphalerite (sph I), galena (gn) and gangues (GGs), binary mixed particles of
gangues with marcasite (GGs/mc) found in particle sizes of +100 mesh. Figure 4. Liberated grains of pyrite (py),
sphalerite (sph I), galena (gn) and gangues (GGs), binary mixed particles of
gangues with marcasite (GGs/mc) found in particle sizes of +100 mesh.
|
Note. The figures show the liberated and interlocked grains in
the study adits.
Source: Prepared by the authors.
Plackett-Burman experimental design for the
Esmeralda, Orión, and Hércules adits
Flotation tests were conducted on representative samples from the three
mine adits under study, focusing on the effects of the reagent blend
(AP-3418/AR-404), AR-242, and the pH of the pulp. The tests evaluated the
following parameters: reagent blend concentrations of 18, 24, and 30 g/t;
AR-242 concentrations of 5, 10, and 15 g/t; and pH values of 10.5, 11.0, and
11.5. In the Esmeralda adit, the highest lead (Pb) recovery was 84.60%, which
was achieved during run order 3. The best grade concentrate achieved was
60.24%, corresponding to run order 6. Additionally, the highest silver (Ag)
recovery was 84.96%, with a head grade of 341.58 oz/t, attained in run order 4.
The results of the total tests for the experimental design of the Esmeralda adit
are detailed in Table 5.
In the Orión adit, the maximum lead (Pb) recovery was 83.73%, achieved in
run order 2, while the highest grade of lead (Pb) concentrate, 61.63%, was
obtained from run order 7. The highest silver (Ag) recovery was 82.50%, achieved
in run order 4, while the best silver (Ag) head grade of 85.35 oz/t was
achieved in run order 3. The experimental design results for the Orión adit are
provided in Table 6.
In the Hércules adit, the best lead (Pb) recovery was 83.79%, as recorded
in run order 9. The highest grade of lead (Pb) concentrate, 56.25%, was
achieved in run order 2. The highest silver (Ag) recovery recorded was 82.57%,
obtained in run order 3, while the best head grade was 63.66 oz/t, noted in run
order 6. The total experimental design tests are summarized in Table 7.
Table 5. Plackett-Burman Experimental Design and Results of the
Metallurgical Tests Conducted at the Esmeralda Adit.
|
Run order
|
pH
|
Reagent blend (AP-3418/AR-404)
|
AR-242
|
pH
|
Reagent blend (AP-3418/AR-404)
|
AR-242
|
Pb recovery (%)
|
Pb head grade (%)
|
Ag recovery (%)
|
Ag head grade (oz/t)
|
|
1
|
1
|
1
|
−1
|
11.5
|
30
|
5
|
83.68
|
57.24
|
84.72
|
297.54
|
|
2
|
1
|
−1
|
−1
|
11.5
|
18
|
5
|
82.00
|
58.66
|
83.79
|
310.64
|
|
3
|
1
|
1
|
1
|
11.5
|
30
|
15
|
84.60
|
56.25
|
84.91
|
326.46
|
|
4
|
0
|
0
|
0
|
11
|
24
|
10
|
83.30
|
56.27
|
84.96
|
341.58
|
|
5
|
0
|
0
|
0
|
11
|
24
|
10
|
83.01
|
56.62
|
84.80
|
339.41
|
|
6
|
−1
|
−1
|
−1
|
10.5
|
18
|
5
|
81.20
|
60.24
|
82.15
|
312.07
|
|
7
|
−1
|
1
|
1
|
10.5
|
30
|
15
|
83.24
|
55.46
|
83.47
|
310.58
|
|
8
|
−1
|
1
|
−1
|
10.5
|
30
|
5
|
82.21
|
59.25
|
81.23
|
295.10
|
|
9
|
−1
|
−1
|
1
|
10.5
|
18
|
15
|
82.36
|
57.78
|
82.06
|
290.00
|
|
10
|
1
|
−1
|
1
|
11.5
|
18
|
15
|
80.77
|
56.80
|
80.82
|
335.07
|
Note. The table presents the operational variables used for
the experimental design.
Source: Prepared by the authors.
Table 6. Plackett-Burman Experimental Design and Results of the
Metallurgical Tests Conducted at the Orión Adit.
|
Run order
|
pH
|
Reagent blend (AP-3418/AR-404)
|
AR-242
|
pH
|
Reagent blend (AP-3418/AR-404)
|
AR-242
|
Pb recovery (%)
|
Pb head grade (%)
|
Ag recovery (%)
|
Ag head grade (oz/t)
|
|
1
|
0
|
0
|
0
|
11
|
24
|
10
|
83.18
|
57.65
|
81.58
|
74.58
|
|
2
|
0
|
0
|
0
|
11
|
24
|
10
|
83.73
|
57.15
|
81.85
|
73.22
|
|
3
|
−1
|
1
|
−1
|
10.5
|
30
|
5
|
81.89
|
61.39
|
81.44
|
85.35
|
|
4
|
−1
|
1
|
1
|
10.5
|
30
|
15
|
82.58
|
59.94
|
82.50
|
84.84
|
|
5
|
1
|
1
|
−1
|
11.5
|
30
|
5
|
82.24
|
58.93
|
81.17
|
77.67
|
|
6
|
−1
|
−1
|
−1
|
10.5
|
18
|
5
|
82.32
|
61.60
|
79.44
|
78.24
|
|
7
|
1
|
−1
|
1
|
11.5
|
18
|
15
|
78.34
|
61.63
|
75.68
|
76.22
|
|
8
|
−1
|
−1
|
1
|
10.5
|
18
|
15
|
83.71
|
59.30
|
81.31
|
77.55
|
|
9
|
1
|
1
|
1
|
11.5
|
30
|
15
|
82.28
|
59.34
|
79.10
|
79.34
|
|
10
|
1
|
−1
|
−1
|
11.5
|
18
|
5
|
78.97
|
60.69
|
78.83
|
76.68
|
Note. The table presents the operational variables used for the
experimental design.
Source: Prepared by the authors.
Table 7. Plackett-Burman Experimental Design and Results of
the Metallurgical Tests Conducted at the Hércules Adit.
|
Run order
|
pH
|
Reagent blend (AP-3418/AR-404)
|
AR-242
|
pH
|
Reagent blend (AP-3418/AR-404)
|
AR-242
|
Pb recovery (%)
|
Pb head grade (%)
|
Ag recovery (%)
|
Ag head grade (oz/t)
|
|
1
|
0
|
0
|
0
|
11
|
24
|
10
|
83.30
|
51.94
|
78.26
|
38.30
|
|
2
|
1
|
−1
|
−1
|
11.5
|
18
|
5
|
82.53
|
56.25
|
79.38
|
36.94
|
|
3
|
−1
|
−1
|
1
|
10.5
|
18
|
15
|
83.29
|
48.11
|
82.57
|
43.38
|
|
4
|
−1
|
1
|
−1
|
10.5
|
30
|
5
|
82.83
|
49.29
|
82.16
|
57.07
|
|
5
|
0
|
0
|
0
|
11
|
24
|
10
|
83.40
|
52.26
|
78.36
|
36.88
|
|
6
|
1
|
1
|
−1
|
11.5
|
30
|
5
|
80.18
|
56.16
|
78.29
|
63.66
|
|
7
|
−1
|
−1
|
−1
|
10.5
|
18
|
5
|
81.31
|
47.17
|
81.82
|
41.29
|
|
8
|
1
|
1
|
1
|
11.5
|
30
|
15
|
82.40
|
54.12
|
80.88
|
62.63
|
|
9
|
−1
|
1
|
1
|
10.5
|
30
|
15
|
83.79
|
48.13
|
80.94
|
55.69
|
|
10
|
1
|
−1
|
1
|
11.5
|
18
|
15
|
84.54
|
54.67
|
80.92
|
37.94
|
Note. The table presents the operational variables used for
the experimental design.
Source: Prepared by the author.
Standardized effects analysis
Table 8 outlines the influence of the variables pH, reagent blend
(AP-3418/AR-404), AR-242, and their interactions on the recovery and
concentrate quality responses for the study adits.
Table 8. Summary of the Interactions of the Main Variables.
|
Adit
|
Response
|
Variables
|
|
Esmeralda
|
Lead (Pb) head grade
|
AR-242
|
|
Lead (Pb) recovery
|
AP-3418/AR-404
|
|
Silver (Ag) head grade
|
pH, (pH – AR-242) interaction
|
|
Silver (Ag) recovery
|
AP-3418/AR-404, pH, (AP-3418/AR-404 – AR-242)
interaction, (pH – AR-242) interaction, (pH – AP-3418/AR-404) interaction
|
|
Orión
|
Lead (Pb) head grade
|
Interaction (pH – AR-242), (pH –
AP-3418/AR-404) interaction, AP-3418/AR-404
|
|
Lead (Pb) recovery
|
Interaction (pH – AR-242), AR-242,
AP-3418/AR-404
|
|
Silver (Ag) head grade
|
AP-3418/AR-404, A, (pH – AP-3418/AR-404)
interaction
|
|
Silver (Ag) recovery
|
pH, AP-3418/AR-404, (pH – AR-242)
interaction
|
|
Hércules
|
Lead (Pb) head grade
|
pH
|
|
Lead (Pb) recovery
|
AR-242, interaction (pH – AP-3418/AR-404)
|
|
Silver (Ag) head grade
|
AP-3418/AR-404
|
|
Silver (Ag) recovery
|
pH, AP-3418/AR-404, (pH – AR-242)
interaction, (pH – AP-3418/AR-404 – AR-242) interaction
|
Note. The table depicts the interactions observed in the
Esmeralda, Orión, and Hércules adits.
Source: prepared by the authors.
Optimization of flotation parameters for polymetallic ore
The independent variables that most significantly contributed to the
flotation process of the three adits under study were optimized using Minitab
19. For the Esmeralda adit, the optimization results indicated a dosage of 18 g/t of reagent blend, 5 g/t of Ar-242, and a pH of 11.5.
For the Orión adit, the optimization results indicated a dosage of 30 g/t of reagent
blend, 5 g/t of Ar-242, and a pH of 10.5. Lastly, for the Hércules adit, the
optimization results indicated a dosage of 30 g/t of reagent blend, 15 g/t of
Ar-242, and a pH of 11.5. The results and optimization plots for the flotation
of minerals from the Esmeralda, Orión, and Hércules adits can be found in
Figures 5, 6, and 7.
|
ESMERALDA ADIT
|
ORIÓN ADIT
|
HÉRCULES ADIT
|
|
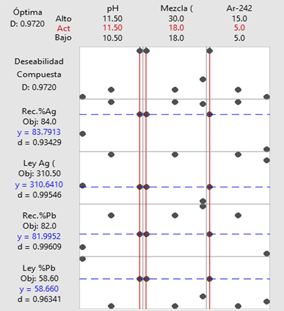
Figure 5. Optimization plot of the
flotation parameters for pit 01.
|
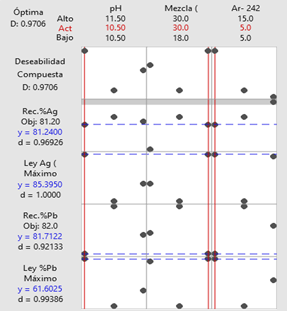 Figure 6. Optimization
plot of the flotation parameters for pit 02. Figure 6. Optimization
plot of the flotation parameters for pit 02.
|
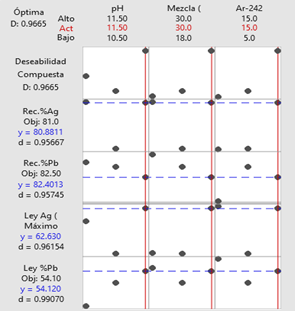 Figure 7. Optimization
plot of the flotation parameters for pit 03. Figure 7. Optimization
plot of the flotation parameters for pit 03.
|
Note. The table presents the optimization plot of the
independent and dependent variables in the three study adits.
Source: Prepared by the authors.
HYPOTHESIS TESTING
The results of the Plackett-Burman experimental design for the Esmeralda adit,
which was developed based on the mineralogical characterization of the adit,
are shown in Table 5. Run order 2 identifies the optimal levels of recovery
and grade of lead-silver concentrate, determined by the dosage of reagents. The
results of test 2 differ from the other runs for this adit in terms of test
conditions, thereby confirming the hypothesis.
For the Orion adit, the experimental design was similarly based on its
mineralogical characterization. The findings indicate that run order 3 achieves
the optimal values for the recovery and grade of the lead-silver concentrate,
which are influenced by the independent variable of reagent dosage. This result
significantly differs from those of the other tests, as illustrated in Table 6,
thus confirming the hypothesis.
Similarly, for the Hercules adit, the development of the experimental
design and selection of reagents for the flotation tests were also based on its
mineralogical characterization. The results from this series of tests
demonstrate that run order 8 yields the optimal values for recovery and grade
of the lead-silver concentrate, as shown in Table 7. These findings differ
significantly from the other runs, further supporting the hypothesis.
DISCUSSION
The results obtained in this research allowed for the optimization of
lead-silver (Pb-Ag) recovery rate by flotation through mineralogical and
metallurgical characterization, as well as the optimal design of the flotation
process of polymetallic ore. The mineralogical characterization revealed that
most valuable particles exhibited a simple intergrowth texture, which proved
beneficial for the concentration process. On the other hand, the experimental
design showed that to enhance metallurgical performance for recovering lead and
silver from the Esmeralda adit, 18 g/t of reagent blend (AP-3418 and AR-404), 5
g/t of AR-242, and an optimal pH of 11.50 were necessary. For the Orion adit,
the optimal parameters included 30 g/t reagent blend (AP-3418 and AR-404), 5
g/t of AR-242, and a pH of 10.50. Similarly, for the Hercules adit, the optimal
parameters were 30 g/t of reagent blend (AP-3418 and AR-404), 15 g/t of AR-242,
and a pH of 11.50.
In comparing the work of Ramos and Orihuela (2017) with this study, which
aimed to evaluate complex polymetallic minerals through flotation metallurgical
tests to obtain copper, lead, and zinc concentrates with satisfactory
recoveries and grades that meet smelting requirements, it was concluded that
the best conditions for copper-lead separation involved a dosage of activated
carbon of 233.3 g/t, 60 g/t of reagent blend BCS, 10 g/t of collector Ap 5100,
and maintaining the pH under natural conditions for 7 minutes. This resulted in
a copper concentrate grade of 25.6% and a lead concentrate grade of 54.3%. In
contrast, this research determined that optimal metallurgical performance in
recovering lead and silver required a reagent blend dosage ranging from 18 to 30
g/t (AP-3418 and AR-404), AR-242 dosages ranging from 5 to 15 g/t, and a pH
level ranging from 10.5 and 11.5, as detailed in Tables 5, 6, and 7.
CONCLUSIONS
The mineralogical characterization of representative
samples from the study adits identified several minerals. In adit 1, the
identified minerals were pyrite, arsenopyrite, galena, chalcopyrite, fahlores,
sphalerite I, magnetite, stibnite, sphalerite II, marcasite, lead sulfosalts,
and pyrrhotite. In adit 2, the minerals found were pyrite, arsenopyrite,
marcasite, galena, chalcopyrite, sphalerite I, sphalerite II, magnetite,
stibnite, hematite, rutile, and fahlores. Finally, in adit 3, the minerals
found were pyrite, arsenopyrite, marcasite, galena, chalcopyrite, pyrrhotite,
sphalerite I, sphalerite II, and rutile. Most of the minerals of interest
exhibited a simple intergrowth texture, while a smaller fraction showed a more
complex intergrowth structure. This complexity can enhance the separation,
flotation, and concentration processes of the minerals of interest.
The standardized analysis of effects in the experimental design allowed
for identifying the interactions of variables (A: pH, B: reagent blend, C:
collector AR-242) that significantly influence the head grade and recovery rate
of lead (Pb) and silver (Ag). Consequently, the parameters for optimizing
metallurgical performance were established as follows: For adit 1, the
recommended dosage is 18 g/t of reagent blend (AP-3418 and AR-404), 5.0 g/t of a
secondary collector AR-242, and a pH of 11.50. For adit 2, the dosage is 30 g/t
of reagent blend (AP-3418 and AR-404), 5.0 g/t of secondary collector AR-242, and
a pH of 10.50. For adit 3, the dosage is 30 g/t of reagent blend (AP-3418 and
AR-404), 15.0 g/t of secondary collector AR-242, and a pH of 11.50.
Operational variables such as particle size, air flow in flotation cells,
and conditioning times contribute to establishing a recovery model and
determining the quality of concentrates from a geometallurgical perspective.
ACKNOWLEDGMENT
We express our gratitude to the Graduate Unit of the School of
Geological, Mining, Metallurgical, and Geographic Engineering at Universidad
Nacional Mayor de San Marcos.
REFERENCES
[1]
Alves, F. E. A. (2014). Caracterização
mineralógica de amostras de Resíduo da Mineração de Chumbo em Boquira (BA) (Degree thesis). Universidade
Federal do Rio de Janeiro, Rio de Janeiro.
https://pantheon.ufrj.br/bitstream/11422/5447/1/ALVES%2c%20F.E.A.pdf
[2]
Aranda Bruno, J. A. (2014). Optimización por
diseño experimental de la flotación de concentrados bulk plomo y plata a nivel
laboratorio en la FIQ. (Degree
thesis). Universidad Nacional del Centro del Perú, Huancayo.
https://repositorio.uncp.edu.pe/bitstream/handle/20.500.12894/3721/Aranda%20Bruno.pdf?sequence=1&isAllowed=y
[3]
Astucuri, V. (1994). Introducción a la
flotación de minerales. Lima N. E.
[4]
Ayllón Meresi, D. E. (2013). Optimización
del proceso de flotación Bulk Plomo-Plata. (Degree
thesis). Universidad Nacional de Ingeniería, Lima.
https://repositorio.uni.edu.pe/handle/20.500.14076/10542
[5]
Bertolino, L., Alves, F., Mendes, J., &
Neumann, R. (2014). Caracterização mineralógica preliminar de amostras do
rejeito da antiga mineração de chumbo em Boquira, Bahia. Comunicações
Geológicas, 101, 965-968. https://www.lneg.pt/wp-content/uploads/2020/03/76_2985_ART_CG14_ESPECIAL_II.pdf
[6]
Bustamante, M. O., Gaviria, A. C., & Restrepo,
O. J. (2008). Notas de clase de la asignatura: Concentración
de minerales. Medellín, Colombia: Universidad Nacional de Colombia.
[7]
Chen, J. (2021). The interaction of flotation
reagents with metal ions in mineral surfaces: A perspective from coordination
chemistry. Minerals Engineering, 171.
https://doi.org/10.1016/j.mineng.2021.107067
[8]
Dou, S., Xu, D., Zhu, Y., & Keenan, R.
(2023). Critical mineral sustainable supply: Challenges and governance. Futures,
146. https://doi.org/10.1016/j.futures.2023.103101
[9]
Elizondo, M., Uribe, A., & Bello, S.
(2021). Chemical stability of xanthates,
dithiophosphinates and hydroxamic acids in aqueous solutions and their
environmental implications. Ecotoxicology and Environmental Safety, 207.
https://doi.org/10.1016/j.ecoenv.2020.111509
[10] Espinoza,
L. A., Iriarte, G., Espinoza, L. O., Gutarra, R., Herrera, M., Zamalloa, J.,
Aramburú, V. S., & Torres, J. A. (2021). Importancia de la mineralogía en
la geometalurgia: aplicación en Perú. Revista del Instituto de investigación
de la Facultad de minas,metalurgia y ciencias geográficas, 24(48), 85-100.
https://doi.org/10.15381/iigeo.v24i48.21707
[11] López
Príncipe, P. H., & Ipanaqué
Nizama, O. S. (2008). Caracterización y optimización de flotación a nivel
laboratorio del mineral de cobre de la minera Candelaria. (Degree thesis). Universidad Nacional Mayor de San Marcos.
https://cybertesis.unmsm.edu.pe/handle/20.500.12672/3272
[12]
Ministerio de Energía y Minas.
(2022). Boletín Estadístico Minero: Por concepto de canon y regalías
mineras, minería genera mayores ingresos para las regiones (Edición N.°
01-2022). Ministerio de Energía y Minas.
https://cdn.www.gob.pe/uploads/document/file/2878178/BEM%2001-2022.pdf.pdf?v=1648163997
[13] Melgarejo,
J. C., Provenza, J. A., Galí, S., & Llovet, X. (2010). Técnicas de
caracterización mineral y su aplicación en exploración y explotación minera. Boletín
de la Sociedad Geológica Mexicana, 62(1), 1-23.
http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1405-33222010000100002&lng=es&tlng=es
[14]
Nassar,
N. T., Graedel, T. E., & Harper, E. M. (2015). By-product
metals are technologically essential but have problematic supply. Science
Advances, 1(3). https://doi.org/10.1126/sciadv.1400180
[15] Ojeda
Escamilla, M. C., Reyes Bahena, J. L., & Aragón Piña, A. (2010, Otober 27-30).
Caracterización mineralógica en la industria minera. Convención Minera
del Bicentenario, Ixtapa, Zihuatanejo, Mexico.
[16] Ramos,
J., & Orihuela, A. (2017). Caracterización y evaluación
de pruebas metalúrgicas de flotación de un mineral complejo polimetálico del
distrito de Palca - Huancavelica.
(Degree thesis). Universidad Nacional Mayor de San Marcos,
Lima. https://alicia.concytec.gob.pe/vufind/Record/UNMS_b2570f1e40152d212efd90f88551c1b6
[17]
Shen, Y., Nagaraj, D. R., Farinato, R., &
Somasundaran, P. (2016). Study of xanthate decomposition in aqueous solutions. Minerals
Engineering, 93, 10-15. https://doi.org/10.1016/j.mineng.2016.04.004
[18]
Song, B., Dong, X., Qiu, X., Hu, Z., &
Wang, Y. (2021). Electronic structure and flotation behavior of Ag-bearing
galena. Journal of Alloys and Compounds, 868. https://doi.org/10.1016/j.jallcom.2021.159105
[19] Taya
Flores, W. H. (2018). Optimización de la flotación polimetálica en la planta
concentradora Mallay. (Degree thesis). Universidad Nacional de San Agustín,
Arequipa.
https://repositorio.unsa.edu.pe/items/f80506e6-7e7f-4c1d-b8c5-b4bb765cb337
[20]
Tercero,
N., Nagaraj, D. R., & Farinato, R. (2019). A
Critical Overview of Dithiophosphinate and Dithiophosphate Interactions with
Base Metal Sulfides and Precious Metals. Mining, Metallurgy &
Exploration, 36, 99-110. https://doi.org/10.1007/s42461-018-0039-1
[21]
Tiu, G., Ghorbani, Y., Jansson, N., &
Wanhainen, C. (2021). Tracking silver in the Lappberget Zn-Pb-Ag-(Cu-Au)
deposit, Garpenberg mine, Sweden: Towards a geometallurgical approach. Minerals
Engineering, 167. https://doi.org/10.1016/j.mineng.2021.106889
[22]
U.S. Geological Survey (2019). Mineral Commodity Summaries 2019.
U.S. Geological Survey. https://doi.org/10.3133/70202434
[23]
Xie, H., Liu, Y., Rao, B., Wu, J., Gao, L.,
Chen, L., & Tian, X. (2021). Selective passivation behavior of galena
surface by sulfuric acid and a novel flotation separation method for
copper-lead sulfide ore without collector and inhibitor. Separation and
Purification Technology, 267.
https://doi.org/https://doi.org/10.1016/j.seppur.2021.118621
[24]
Yekeler, M., & Yekeler, H. (2006). A
density functional study on the efficiencies of 2-mercaptobenzoxazole and its
derivatives as chelating agents in flotation processes. Colloids and
Surfaces A: Physicochemical and Engineering Aspects, 268(1-3), 121-125.
https://doi.org/10.1016/j.colsurfa.2006.03.012
[25]
Yovanovic, A. (2004). Engenharia da
Concentração de Massa por Flotação. Belo Horizonte, MG, Brazil: Modelo
Operacional.
https://www.modelooperacional.com.br/wp-content/uploads/2019/04/Engenharia-da-Concentracao-de-Massa-por-Flotacao.pdf
[26]
Zou, S., Lin, Q., Wang, S., Ma, X., &
Zhong, H. (2022). A novel surfactant O,O'-bis(2-butoxyethyl)
ammonium dithiophosphate: Synthesis, selective flotation and adsorption
mechanism towards galena. Minerals Engineering, 179.
https://doi.org/https://doi.org/10.1016/j.mineng.2022.107466
Authors’ contribution
José David Valverde Díaz (first author): Investigation, funding
acquisition, project administration, and software.
Vidal Sixto Aramburú Rojas (co-author): Conceptualization, data curation,
formal analysis, and resources.
Jorge Alberto Ortiz Barreto (co-author): Investigation, methodology, visualization,
original draft preparation, and writing (review & editing).
Rosa María Tiburcio Alva (co-author): Formal analysis, methodology, supervision,
and validation.
Sharon Elisa Aguilar Zevallos: Investigation and writing (review &
editing).






